Addition Of Bromine (Br2) To Alkenes Is Stereoselective, Giving “Anti” Addition Stereochemistry
- Get link
- X
- Other Apps
Let’s look at a different reaction next. When we treat an alkene with a halogen such as Br2, (often in a halogenated solvent such as CH2Cl2 or CCl4) we obtain the following product using 1,2-dimethylcyclohexene.
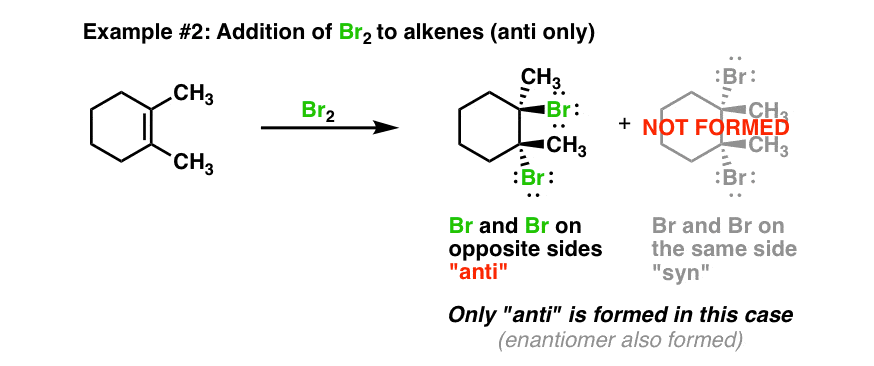
Again, pay attention to the dashes and wedges. Here, notice that we observe only the “anti” product and none of the “syn” product. In other words, the reaction is highly selective for one stereoisomer over the other. We could go even further and say that because of the complete absence of the “syn” product, the reaction is stereospecific for the “anti”. Only one type of stereoisomer is formed.
We’ll see that this pattern is observed for other reactants similar to Br2. Again, any mechanism we propose will have to account for the fact that we only get the “anti” product and none of the “syn”.
- Get link
- X
- Other Apps


Comments
Post a Comment
Thanks