SUSTAINED RELEASE DOSAGE FORM
The oral route of drug delivery is typically considered the preferred and most patient-convenient means of drug administration. With many drugs the basic Goal of therapy is to achieve a steady-state blood or tissue level that is therapeutically effective and nontoxic for an extended period of time. Sustain release system are considered a wiser approach for the drugs with short half-lives and which require repeated dosing, they are easy to formulate and are irrespective of absorption process from gastrointestinal tract after oral administration. The basic objective of these dosage forms is to optimize the delivery of medications so as to achieve a measure of control on therapeutic effect in the face of uncertain fluctuations in the in vivo environment in which drug release takes place. The advances in the formulation technology of modified release dosage form with sustained release oral dosage form has been widely accepted approach as compared to conventional immediate release formulations of the same drug, over which it provides a prolong release of the drug over extended period of time there by giving the better patient compliance and enhanced bioavailability and resulting blood concentration-time profiles of drugs that otherwise suffer from few limitations.
INTRODUCTION: -
Traditional drug delivery system has been characterized by immediate release and repeated dosing of the drug which might lead to the risk of dose fluctuation, this arises the need of a formulation with control release that maintain a near-constant or uniform blood level. The desire to maintain a near-constant or uniform blood level of a drug often translates into better patient compliance, as well as enhanced clinical efficacy of the drug for its intended use.
Drawbacks of Conventional Dosage Forms: -
1. Poor patient compliance, increased chances of missing the dose of a drug with short half-life for which frequent administration is necessary.
2. The unavoidable fluctuations of drug concentration may lead to under medication or over medication.
3. A typical peak-valley plasma concentration time profile is obtained which makes attainment of steady-state condition difficult.
4. The fluctuations in drug levels may lead to precipitation of adverse effects especially of a drug with small Therapeutic Index (TI) whenever over medication occur.
Sustained release concept: - Sustained release, sustained action, prolong action, controlled release, extended action, depot are terms used to identify drug delivery systems that are designed to achieve prolong therapeutic effect by continuously releasing medication over an extended period of time after administration of single dose. In the case of orally administer this period is measured in hours while in the case of injectables this period varies from days to months.
[adsense:468x15:2204050025]
Advantages of sustained release dosage forms:-
1. Control of drug therapy is achieved.
2. Rate and extent of drug absorption can be is modified
3. Frequency of drug administration is reduced.
4. Patient compliance can be improved.
5. Drug administration can be made convenient
6. Maximizing the availability of drug with minimum dose.
7. The safety margin of high potency drug can be increased.
Disadvantages of sustained release dosage forms: -
1. It not permits prompt termination of therapy.
2. Less flexibility in dose adjustment.
3. These dosage forms are designed on the basis of average biological half life.
4. They are costly.
PARAMETERS FOR DRUG TO BE FORMULATED IN SUSTAINED RELEASE DOSAGE FORM:
Physicochemical parameters for drug selection.
1. Molecular weight/size 1000 Daltons.
2. Solubility > 0.1 mg/ml for pH 1 to pH 7.8.
3. Apparent partition coefficient High.
4. Absorption mechanism Diffusion.
5. General absorbability from all GI segments.
6. Release should not be influenced by pH and enzymes.
Pharmacokinetic parameters for drug selection
1. Elimination half-life preferably between 2 to 8 hrs
2. Total clearance should not be dose dependent
3. Elimination rate constant required for design
4. Apparent volume of distribution (Vd) The larger Vd and MEC, the larger will be the required dose size
5. Absolute bioavailability should be 75% or more
6. Intrinsic absorption rate must be greater than release rate
7. Therapeutic concentration Css The lower Css and smaller Vd, the loss among of drug required.
8. Toxic concentration Apart the values of MTC and MEC, safer the dosage form. Also suitable for drugs with very short half-life.
FACTORS AFFECTING THE ORAL SUSTAIN RELEASE DOSAGE FORM DESIGN
A) Pharmacokinetics and pharmacodynamics factor:
1. Biological half-life
Drug with biological half-life of 2-8 hours are considered suitable candidate for sustain release dosage form, since this can reduce dosing frequency. However this is limited in that drugs with very short biological half lives may require excessive large amounts of drug in each dosage unit to maintain sustained effects, forcing the dosage form itself to become limitingly large.
2. Absorption
Rate of absorption of a sustained formulating depends upon release rate constant of the drug from the dosage form, and for the drugs that are absorbed by active transport the absorption is limited to intestine.
3. Distribution
The distribution of drugs into tissues can be important factor in the overall drug elimination kinetics. Since it not only lowers the concentration of circulating drug but it also can be rate limiting in its equilibrium with blood and extra vascular tissue, consequently apparent volume of distribution assumes different values depending n the time course of drug disposition. Thus for design of sustain release products, one must have information of disposition of drug.
4. Metabolism
The metabolic conversion to a drug is to be considered before converting into another form. Since as long as the location, rate, and extent of metabolism are known a successful sustain release product can be developed.
B) Drug properties relevant to sustain release formulation:
1. Dose size
A dose size of 500-1000mg is considered maximal for a conventional dosage form. This also holds true for sustain release dosage forms. Since dose size consideration serves to be a parameter for the safety involved in administration of large amounts with narrow therapeutic range.
2. Ionization, pka and aqueous solubility
Most drugs are weak acids or bases and in order for a drug to get absorbed, it must dissolve in the aqueous phase surrounding the site of administration and then partition into the absorbing membrane.
3. Partition coefficient
Bioavailability of a drug is largely influenced by the partition coefficient, as the biological membrane lipophilic in nature transport of drug across the membrane largely depends upon the partition coefficient of the drug. Drugs having low partition coefficient are considered as poor candidate for the sustain release formulation as it will be localized in the aqueous phase eg: Barbituric acid and vice a versa.
4. Drug stability
When drugs are orally administered, they come across acid-base hydrolysis and enzymatic degradation. In this case, if the drug is unstable in stomach, drug release system which provides medication over extended period of time is preferred, whereas in contrast the drug unstable in intestine will face problem of less bioavailability
DESIGN OF ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEM: -
The oral route administration is mostly adopted route because of its comfortable dosage form, design and patient care. Several parameters should be kept in mind before formulating sustain release dosage form which includes various pH in GIT, the gastrointestinal motility, the enzyme system and its effect on the dosage form and the drug. Most of sustained release dosage form follows the mechanism of diffusion, dissolution or combination of both, to produce slow release of drug at predetermined rate. Hypothetically, a sustained release dosage form should release the drug by a zero-order mechanism which maintains drug plasma level time similar to intravenous infusion. Plasma drug concentration-profiles for conventional tablet or capsule formulation, a sustained release formulation, and a zero order sustained release formulation are as follow in given figure
Figure 2: Comparison of conventional and controlled release profiles.
APPROACHES TO SUSTAIN RELEASE DRUG DELIVERY SYSTEM
1. Dissolution controlled release systems.
2. Diffusion controlled release systems.
3. Dissolution and diffusion controlled release systems.
4. Ion exchange resin- drug complexes.
5. pH dependent formulation.
6. Osmotic pressure controlled systems.
1. Dissolution controlled release systems
These systems are easy to formulate. Drug which are formulated using system have slow dissolution rate, produce slow dissolving forms with gastric intestinal fluids and the drugs which are having high aqueous solubility and dissolution rate. Dissolution controlled release system can be classified into two techniques
A. Matrix dissolution controlled release system
Matrix dissolution system is known as monolithic because the drug present in the matrix is completely dissolved in the medium which controls the drug release. They are mostly made of waxes like beeswax, carnauba wax, hydrogenated castor oil, etc. and play important role to control the drug release rate by controlling the rate of dissolution fluid penetration into the matrix by altering the porosity of tablet, decreasing its wettability or by itself getting dissolved at a slower rate The drug release generally follows first order kinetics from such matrices system.
B. Reservoir dissolution controlled release system
In reservoir system, the drug particles are coated or encapsulated with one of the several microencapsulation techniques using slowly dissolving materials like cellulose, polyethylene glycol and waxes. This unit can be encapsulated in capsules or may be compressed into tablets Solubility and thickness of the coating play important role in dissolution rate of drug.
2. Diffusion controlled release systems
In diffusion release models, the diffusion of dissolved drug through a polymeric membrane is a rate limiting step. In this system, the drug release rate never follows zero-order kinetics, because the diffusion path length increases with time as the insoluble matrix is drug depleted. The mechanism of diffusion process shows the movement of drug molecules from a region of a higher concentration to region of lower concentration. The flux of the drug J (in amount / area -time), across a membrane in the direction of decreasing concentration is given by Fick’s law.J = -D dc/dx where, J = flux of the drug across a membrane in the direction of decreasing conc.,D = Diffusion coefficient of the drug, and dc /dx = Change in the concentration of the drug in the membranewhereas when drug present in a water insoluble membrane, it must diffuse through the membrane.The drug release rate dm/ dt is given by dm = ADKΔ C/dt L where, A = Area. K = Partition coefficient of drug between the membrane and drug core. L = Diffusion path length (i.e. thickness of coat). ΔC=Concentration difference across the membrane.
3. Dissolution and diffusion controlled release systems
In this kind of system, the drug is enclosed in a membrane which is partially water soluble. The dissolution of the membrane take place due to which pores are formed and these pores allows aqueous medium to enter in the membrane. This results in the dissolution of the drug in membrane followed by the diffusion of the dissolved drug from the system. Example of such coating is combination of ethyl cellulose with PVP or methyl cellulose.
4. Ion exchange resin- drug complexes:
Resins are the materials which are insoluble in water. Resin contains anionic groups such as amino or quaternary ammonium groups and cationic groups such as carboxylic groups, or sulfonic groups in repeating positions on the chain. A drug–resin complex is formed by prolonged exposure of drug to the resin. The drug from these complexes gets exchanged in gastrointestinal tract and later they are released with excess of Na+ and Cl- present in gastrointestinal tract.
Resin+ – Drug– + Cl– ----------- > >> resin+ Cl– + Drug–
Where x- is Cl- conversely
Resin– – Drug+ + Na+ ----------- > >> resin– Na+ + Drug
Water insoluble cross linked polymer compounds are used for this system.
5. PH dependent formulation
Some drugs on dissolution and absorption in GIT, changes the pH present in the gastrointestinal tract, so dosage forms are formulated using sufficient amount of buffering agent like salt of phosphoric, citric or tartaric acids. These salts adjust the pH to the desired value when dosage form move across the gastrointestinal tract. Permeable coating agents are used to coat the drug and buffer present in the dosage form, which allows the aqueous medium to enter in it and prevents the dispersion of the tablets.
6. Osmotic pressure controlled systems
These types of system are also known as oros, which follows the mechanism of osmotic pressure where the drug is released at constant zero order rate. The reservoir is made up of the drug and osmotic agent like mannitol or KCl, which is surrounded by semi permeable membrane. A small orifice is present in the dosage form, which allows the entry of water in the reservoir and helps the dissolved drug to pumped out at the determined rate due to osmotic pressure. The release of the drug from the reservoir is unaffected by the conditions of the GIT. The release of drug is depended on factors like size of orifice, thickness of semi permeable membrane, permeability of membrane, osmotic properties of core and stability of the drug.
Evaluation of sustained release tablet dosage form:-
1. Weight variation: Twenty tablets were randomly selected from each batch individually weigh, the average weight and standard deviation of 20 tablets calculated.
2. Thickness: The thickness of the tablet was measured by using digital venire caliper, twenty tablets from each batch were randomly selected and thickness was measured.
3. Hardness: Hardness was measured using Pfizer hardness tester, for each batch three tablet were tested.
4. Friability: Twenty tablets were weight and placed in the Roche friabilator and apparatus was rotated at 25 rpm for 4 min. After revolution the tablets were dusted weight.
5. Drug content uniformity: - It is determined by means of the Assay procedures.
6. In-Vitro Dissolution Study: -These studies vary according to the drug employed in the formulation this example is of Nicorandil sustained release matrix tablet. The study was carried out using 0.1NHcl andphosphate buffer 7.4 using the USP apparatus types II,the dissolution medium 900 ml maintained at 37oc ±0.5oc, the absorbance was measured at 262nm, thedissolution study were carried out for 24 hrs.
CONCLUSION:
Sustain release system are considered a wiser approach for the drugs with short half-lives and which require repeated dosing, they are easy to formulate and are irrespective of absorption process from gastrointestinal tract after oral administration. For the formulation of sustained release dosage forms it need good process development. Besides their prominent advantages over conventional dosage forms they suffer from the drawbacks like less flexibility is dosage adjustment, costly, do not permit prompt termination of therapy. This concept, however, requires accurate adjustment of the physicochemical parameters of core material, coating formulation and tableting excipients. Many drugs are formulated as sustained release dosage form to achieve a prolonged therapeutic effect by continuously releasing medication over an extended period of time after administration of single dose of drug. Hence, sustained release drug delivery system is the preferred dosage form for the drugs having short half-life, so as to maintain the drug plasma level in therapeutic index for prolonged period of time.
REFERENCES:
1. Lachman L., Lieberman, H. A., Joseph L. K. The Theory and Practice of Industrial Pharmacy; Varghese Publishing House; Mumbai; Third Edition; Pp .430-456.
2. Aulton E. Micheal. Modified release per oral dosage form, Pharmaceutics-The science of dosage form design, New York: Churchill living stone; 575.
3. Banker SG, Rhodes TC. Modern Pharmaceutics. Marcel Dekker INC, New York: 2002; 575.
4. Donald LW. Handbook of pharmaceutical controlled release technology. Marcel Dekker INC. New York: 2000; 432-480.
5. Bramhankar HA, Jaiswal SB. Biopharmaceutics and pharmacokinetics A treatise. Vallabh prakashan 2000: 348-357.
6. Bhargava Ankit, Rathore R.P.S., Tanwar Y.S., Gupta S, Bhaduka G. oral sustained release dosage form: an opportunity to prolong the release of drug IJARPB. 2011; 3(1), 7-14.
7. Ansel HC, Allen LV, and Popovich NG, Pharmaceutical dosage forms and drug delivery system, 9thedition, Lippincott William & Wilkins, 2005, 257-271.

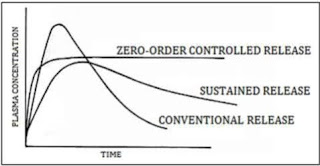 Figure 1:
Figure 1: 

Comments
Post a Comment
Thanks