Pre formulation Studies
DEFINITION:-Investigation of physico-chemical properties of the new drug compound
that could affect drug performance and development of an efficacious dosage
form”.Preformulation commences when a newly synthesized drug shows a sufficient
pharmacologic promise in animal model to warrant evaluation in man.
INTRODUCTION
The preformulation is
the first step in the rational development of a dosage form of a drug substance
alone and when combined with excipients.
Objective :
To generate useful
information (physico-chemical , kinetics, stability and compatibility with
excipients) to the formulator to design an optimum drug delivery system.
Before embarking on a
formal programme of preformulation, scientist must consider the following :
1. Available
physicochemical data (including chemical structure, different salt available)
2. Anticipated dose
3. Supply situation
and development schedule
4. Availability of
stability – indicating assay
Organoleptic properties
Color:
Color is generally a
function of a drug’s inherent chemical structure relating to a certain level of
unsaturation (double or triple bond).Color intensity relates to the extent of
conjugated unsaturation as well as the presence of chromophores.Some compound
may appear to have color although structurally saturated.
Odor
·
The substance may
exhibit an inherent odor characteristic of major functional groups present.
·
Odor greatly affects
the flavor of a preparation or food stuff
Taste
·
If taste is considered
as unpalatable, consideration is to be given to the use of a less soluble
chemical form of the drug.
·
The odor and taste may be
suppressed by using appropriate flavors and excipients or by coating the final
product.
PURITY
Designed to estimate
the levels of all known & significant impurities & contaminates in the
drug substance under evaluation. Study performed in an analytical research
& development group.It is another parameter which allows for comparison
with subsequent batches.
The techniques used
for characterizing the purity of a drug are the same as those used for other
purpose in a preformulation study.
Thin layer chromatography
is a wide ranging applicability & is an excellent tool for characterizing
the purity. HPLC, paper chromatography & gas chromatography are also
useful. More quantitative information can be obtained by using quantitative
differential scanning colorimetry.
PARTICLE
SIZE
Particle size is characterized using these terms :
Very coarse (#8)
Coarse (#20)
Moderately coarse
(#40)
Fine (#60)
Very fine (#80)
Particle size can
influence variety of important factors :
- Dissolution rate
- Suspendability
- Uniform distribution
- Penetrability
- Lack of grittiness
SURFACE AREA
Particle size &
surface area are inversely related to each other.Smaller the drug particle,
greater the surface area.Specific surface is defined as the surface area per
unit weight (Sw) or unit volume (Sv) of the material
HOWEVER SIZE REDUCTION
IS NOT REQUIRED IN FOLLOWING CASES:
WHEN DRUG IS UNSTABLE.
DEGRADE IN SOLUTION
FORM.
PRODUCE UNDESIRABLE
EFFECTS.
WHEN SUSTAINED EFFECT
IS DESIRED
Particle Shape
Particle shape will
influence the surface area, flow of particles, packing & compaction
properties of the particles.
A sphere has minimum
surface area per unit volume.
Therefore, these
properties can be compared for spheres & asymmetric particles, in order to
decide the shape.
SOLUBILITY
The amount of
substance that passes into solution in order to establish equilibrium at
constant temperature and pressure to produce a saturated solution.
If solubility is
<1mg/ml indicates need for salt formation to improve solubility.
If solubility is
<1mg/ml in pH= 1 to 7, preformulation study should be initiated.
Solubility should
ideally be measured at two temperatures: 4°C and 37°C.
·
4°C to ensure Physical
stability.
·
37°C to support
Biopharmaceutical evaluation.
DESCRIPTIVE SOLUBILITIES
|
Description
|
Parts
of solvent |
|
Very
soluble |
1 |
|
Freely
soluble |
1-10 |
|
soluble |
10-30 |
|
Sparingly
soluble |
30-100 |
|
Slightly
soluble |
100-1000 |
|
Very
slightly soluble |
1000-10,000 |
|
Insoluble |
>
10,000 |
SOLUBILITY
ANALYSIS
Analytic methods that
are particularly useful for solubility measurement include HPLC, UV
spectroscopy, Fluorescence spectroscopy and Gas chromatography. Reverse phase
HPLC offer accurate and efficient mean of collecting solubility data of drug.
Ionization constant (pKa)
Can be calculated by Henderson Hasselbach equation-For acidic
drugs….pH= pKa+ log [ionized drug]/[unionized drug]
For basic drugs….pH=
pKa+ log[unionized drug]/[ionized drug]
pH Solubility Profile
The solubility of
acidic or basic drug will show difference with changes in pH.So according to
general role (salt formation), solubility of basic drugs will increased as the
pH decreased, and the solubility of acidic drugs increased as the pH
increased.pH solubility profile of a drug can be established by running the
equilibrium solubility experiment within pH range of 3-4.
Partition Coefficient
It is the ratio of unionized drug distributed between organic
and aqueous phase at equilibrium. It’s a measurement of a drug's lipophilicity
and its ability to cross the cell membrane.
P o/w = ( C oil / C
water )equilibrium
Effect
Of Temperature
The heat of solution ∆Hs, represents the heat released or absorbed
when a mole of solute is dissolved in large quantity of solvent.
most commonly the
solution process is Endothermic reaction (+) , thus by increasing the temp. the
solubility will be increased.
Exothermic reaction
(-) for such solutes as lithium chloride and other HCL salts that are ionized
when dissolved ,thus increasing the temp. will decrease the solubility.
Determination of solubility
The following points should be considered:
-The solvent &
solute must be pure.
-A saturated solution
must be obtained before any solution is removed for analysis.
-The method of
separating a sample of saturated solution from undissolved solute must be
satisfactory.
-The method of
analyzing solution must be reliableTemperature must be adequately controlled .
Solubility Determination Method
-Solubility is normally depends on temperature, so temperature
is recorded in each solubility measurement.
- Plot of solubility
against temperature is commonly used for solubility determination.
- Two methods are
available for determination are as follow.
1.
Synthetic method
2. Analytical method: Temp. of equilibrium is fixed and conc. Of the
solute in the saturated sol. Is determined at equilibrium by a suitable
analytical procedure.
General
Methods of Increasing the Solubility (Solubilization)
Addition of co-solventSalt formationReduction of particle
sizeThe choice of proper polymorphProdrug approachAddition of
SurfactantpH-change of solutionTemp.effectComplexation
Process Of Solubilization
The process of solubilization involves the breaking of
inter-ionic or intermolecular bonds in the solute, the separation of the
molecules of the solvent to provide space in the solvent for the solute,
interaction between the solvent and the solute molecule or ion.
Step 1: Holes
opens in the solvent DEPT. OF PHARMACEUTICS
Step2: Molecules of the solid breaks away from the bulk
Step 3:
The free solid molecule is intergraded into the holes in the solvent
SOLUBILIZATION
When surfactants are added to
the liquid at low concentration they tend to orient at the air-liquid interface
.On further addition of surfactant the interface becomes completely occupied
and excess molecules are forced into the bulk of liquid.At very high
concentration surfactant molecules in the bulk of liquid begin to form micelles
and this concentration is know as CRITICAL MICELLECONCENTRATION{CMC}DEPT. OF PHARMACEUTICS
Solubilization is thought to
occur by virtue of the solute dissolving in or being adsorbed onto the
micelle.Thus the ability of surfactant solution to dissolved or solubilize
water insoluble materials starts at the CMC and increase with increase in the
concentration of micelles.Solubilization of any material in any solvent depends
on proper selection of solubilising agents.
Addition Of Co-Solvent
Weak Electrolyte :- PhenobarbitoneNon polar :- Nitro
CelluloseThese are poorly soluble in given solvent.For such poorly soluble
materials, to enhance their solubility, the water miscible solvents are used in
which the drug has good solubility.This process of improving solubility is
known as co-solvency and the solvent used is known as co-solvents.
e.g. Phenobarbitone is insoluble in water. A clear solution is
obtained by dissolving it in a mixture of Alcohol, Glycerin, Propylene
glycol.e.g. Of Cosolvents:-PG, glycerin, sorbitol, PEG, Glyceryl formal,
glycofurol, ethyl carbamate, ethyl lactate and dimethyl acetamide.
pH change Method Weak base:- Alkaloids, Local Anaesthesia
Weak acid:- Sulphonamides, BarbituratesIn aqueous medium they
dissociate poorly and undissociated portion is insoluble.e.g. Benzoic acid,
PhenobarbitoneSo, solubility of the undissociated portion is improved by pH
control.For weak acidic drug:- increase pH, solubility is increase.For weak
base drug:- decrease pH, increase solubility.
Reduction Of Particle size
Reduction in Particle size improve solubility of drug.Basically
reduction in particle size increase contactsurface area of the particle, there
by ultimately it increase rate of solubility of drug.
Applications of solubilization
Drugs with limited aqueous solubility can be solubilized. These
include oil-soluble vitamins, steroid hormones and antimicrobial agents etc.
Solubilization of orally administered drugs results in an improved appearance and improves unpleasant taste.Both oil-soluble and water-soluble compounds can be combined in a single phase system as in case of multivitamin preparations.
Solubilization may lead to enhanced absorption and increased
biological activity.Improves the intestinal absorption of vitamin A.
Drug absorption from ointment bases and suppositories also increased.Liquid preparations with small quantity of preservative can be prepared by solubilization.
Aqueous concentrates of volatile oils can be prepared by
solubilization.
Example: soaps used
for solubilising phenolic compounds for use as disinfectants- Lysol, Roxenol
etc.
Barbiturates,
anticoagulant, alkloidal drugs are dissolved with polysorbate by
solubilization.
Crystallinity
Crystal habit &
internal structure of drug can affect bulk & physicochemical properties of
molecule.
Bulk prop. Like hygroscopocity,
flowabilty and density.Crystal habit is description of outer appearance of
crystal.
Internal structure is
molecular arrangement within the solid.
Crystallinity and Polymorphism
There are two main classes of the solids: crystalline and
amorphous.
what distinguishes
them is the nature of their atomic scale structure.
Atomic positions in
crystal exhibit a property called long-range order positions repeated in space
in regular array.
Amorphous material is a random
Arrangement of the
molecules .
Different shapes of crystals
Cubic or isometric -
not always cube shaped. Also find as octahedrons (eight faces) and
dodecahedrons (10 faces).
Tetragonal-
similar to cubic crystals, but longer along one axis than the other, forming
double pyramids and prisms.
Orthorhombic - like
tetragonal crystals except not square in cross section (when viewing the
crystal on end), forming rhombic prisms or dipyramids (two pyramids stuck
together).
Hexagonal -
six-sided prisms. When you look at the crystal on-end, the cross section is a
hexagon.
Trigonal - possess a single
3-fold axis of rotation instead of the 6-fold axis of the hexagonal division.
Triclinic -
usually not symmetrical from one side to the other, which can lead to some
fairly strange shapes.
Monoclinic - like skewed tetragonal crystals, often forming prisms and double pyramids.
Solubility & dissolution rate are greater for amorphous form
then crystalline, as amorphous form has higher thermodynamic energy. Also
amorphous solubility is higher because of its larger surface area, but at the
same time stability is low and its more affected by PH and its factor.
Amorphous powder with storage convert from unstable to stable form.
Amorphous form
typically prepared by :
Lyophilization, rapid
precipitation, and rapid cooling of the liquid melts.
When water incorporated in the structure , the complex called hydrate.
If it contains one molar equivalent of H2O , called
monohydrate.Contains two molar of H2O , called dihydrate.Contains no
water, called anhydrous.Hydrate compounds are important since their aqueous
solubility are significantly less than that of anhydrous form. Conversion of
anhydrous to hydrate within the dosage form reduce the solubility and
dissolution rate of the drug like anhydrous and trihydrate forms of Ampicillin
.
Polymorphism
It is the ability of
the compound to crystallize as more than one distinct crystalline species of
the same pure substance with different internal lattice.Different crystalline
forms are called polymorphs.
Physical properties
differ among various polymorphs:
Molar vol. and density
Refractive index
Melting and
sublimation temp.
Enthalpy (heat
content)
Solubility
Dissolution rate
Stability
Hardness
Compatibilty ,
handling, flow and blending.
During preformulation
it is important to identify the polymorph that is stable at room
temp.Chloramphenicol exist in A,B & C forms, of these B form is more
soluble & most preferable as the serum level of it is higher but at the
same time it is less stable and may converted to stable form upon storage.The
least stable polymorph has more solubility and vice versa.
Techniques
for studies of crystals
Microscopy
Hot stage microscopy
Thermal analysis
X-ray diffraction
Microscopy
Material with more
than one refractive index appears bright with brilliant colors against black
polarized background.The color intensity depends upon crystal
thickness.materials havesingle refractive indexand this substance do
nottransmit light with crossedpolarizing filter and appearsblack.
Advantage :
By this method, we can study crystal morphology & difference
between polymorphic form.
Disadvantage :
This require a well
trained optical crystallographer, as there are many possible crystal habit
& their appearance at different orientation.
Hot stage microscopy
The polarizing
microscope fitted with hot stage is useful for investigating polymorphism,
melting point & transition temp.
Disadvantage :In
this technique, the molecules can degrade during the melting process.
DSC And Thermal analysis
Differential scanning calorimetry (DSC) & Differential
thermal analysis are (DTA) are particularly useful in the investigation of polymorphism.
·
It measures the heat
loss or gain resulting from physical or chemical changes within a sample as a
function of temp.
·
Disadvantage : Degradation
during thermal analysis mayprovide misleading results.
X-ray diffraction
Working :
When beam of nonhomogenous X-ray is allow to pass through the
crystal, X-ray beam is diffracted & it is recorded by means of photographic
plate.Diffraction is due to crystal which acts as 3 dimensional diffraction
grating toward X-ray. Random orientation of crystal lattice in the powder
causes the X-ray to scatter in a reproducible pattern of peak intensities.The
diffraction pattern is characteristic of a specific crystalline lattice for a
given compound. An amorphous form does not produce a pattern mixture of
different crystalline forms.



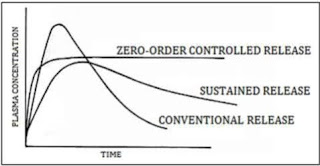
Comments
Post a Comment
Thanks